
Predation on Ostracods
Ostracods can be consumed by chance (e.g. by water birds foraging in sediments and water plants), or by animals actively seeking them. Typically, ostracods make up a small part of the diet of predators, but some predators, including the siphonophore Hippopodius hippopus and some species of water mites, feed exclusively on ostracods (Purcell 1981; Proctor & Pritchard 1989).
The consumption of ostracods can affect the capability of the midshipman fish of the Pacific coast of North America to bioluminesce. Those that live in the same area as bioluminescent ostracods and hence can eat them are able to produce light from the 700+ small dermal photophores on their heads and trunks. Those fish further north where bioluminescent ostracods do not live, do not bioluminesce. However, if northern midshipman fish are fed bioluminescent ostracods, they too can emit light (Thompson & Tsuji 1989).

A bluegill (Lepomis macrochirus), a predator of ostracods.
| Predator | Notes |
|---|---|
| Fish (Osteichthyes) | A wide variety of both marine and freshwater fish have been reported to prey on ostracods (e.g. Harding 1962; Victor et al. 1979; Vinyard 1979; Allen & Wootton 1984; Gordon et al. 1985; Morin 1986; Whatley, in Henderson 1990; Mbahinzireki et al. 1991; Griffiths et al. 1993; Uiblein et al. 1994; Aarnio & Bonsdorff 1997; Aarnio & Mattila 2000; Blanco et al. 2004). |
| Waterfowl (Aves, Anseriformes) | Ostracods have been recovered from the guts of teal, while viable ostracod eggs have been recovered from the guts of mallards, teal and gadwalls. These were probably ingested by chance (Proctor 1964; Brochet et al. 2010). |
| Turtles (Reptilia, Testudines) | Ostracods occur in the varied diets of some freshwater turtles (Ernst & Lovich 2009; Alcalde et al. 2010). |
| Rhynchosaurs? (extinct) (Reptilia, Rhynchosauria) | A Triassic reptilian coprolite, possibly from a rhynchosaur, contained ostracods. These were probably ingested by accident (Sohn & Chatterjee 1979). |
| Frogs and tadpoles (Amphibia, Anura) | Elpidium have been reported to be predated on by tadpoles of snouted treefrogs, but pass through the gut unharmed. Live ostracods have also been retrieved from the rectum of a frog (Lowndes 1930; Lopez et al. 2002). |
| Salamanders and newts (Amphibia, Caudata) | Ostracods can be the most important prey for larval stages of slamanders and newts (Brophy 1980; Spencer & Blaustein 2001; Ghioca-Robrecht & Smith 2008). |
| Naticidae and Muricidae gastropods (Mollusca, Gastropoda) | These marine snails predate ostracods by drilling a hole through the carapace. Fossil evidence indicates this type of predation on ostracods has occurred for over 100 million years (Reyment 1966; Maddocks 1988; Reyment & Elewa 2003). |
| Poromyidae bivalves (Mollusca, Bivalvia) | Although sessile, these bivalves have been found with the remains of ostracods in their guts (Leal 2008). |
| Dragonfly and damselfly larvae (Insecta, Odonata) | Larvae of the emperor dragonfly dramatically reduced population densities of Heterocypris incongruens (Havel et al. 1993; Vandekerkhove et al. 2012). |
| Alderfly larvae (Insecta, Megaloptera) | In a study, it was reported that Sialis itasca feeds almost exclusively on the ostracod Cyclocypris sp. (Lilly et al. 1978). |
| Non-biting midge larvae (Insecta, Diptera, Chironomidae) | Predatory chironomid larvae consume ostracods as well as other groups. A study showed that ostracods will be eaten by non-biting midge larvae, but other food is preferred (Baker & McLachlan 1979; Vodopich & Cowell 1984). |
| Mosquito larvae (Insecta, Diptera, Culicidae) | Ostracods can be the most consumed prey of mosquito larvae in some habitats (Campos & Lounibos 2000). |
| Caddisfly larvae (Insecta, Trichoptera, Hydropsychidae) | Ostracods can feature in the diet of caddisflies (Tszydel & Grzybkowska 2011). |
| Backswimmers (Insecta, Hemiptera, Notonectidae) | Pigmy backswimmers, and to a lesser extent common backswimmers can dramatically reduce the population densities of Heterocypris incongruens (Vandekerkhove et al. 2012). |
| Water boatmen (Insecta, Hemiptera, Corixidae,) | Ostracods feature in the varied diets of water boatmen (Papácek 2001). |
| Aquatic beetles (Insecta, Coleoptera, Dytiscidae) | Besides other groups (e.g. copepods and mosquito larvae), aquatic beetles also predate on ostracods. An experiment demonstrated that for one beetle species, mosquito larvae are preferred to ostracods. (Arnold & Convey 1998; Culler & Lamp 2009). The larvae of one species has specialized mouth parts for predation on ostracods, facilitated by a projection on the head (Hayashi & Ohba 2018). |
| Flatworms (Platyhelminthes, Turbellaria) | Both Cypridopsis vidua and Physocypria pustulosa are eaten by flatworms. Juvenile Darwinula stevensoni have been reported to survive attempted predation by flatworms (Legner et al. 1976; Smith & Kamiya 2008). |
| Oligochaetes (Annelida, Oligochaeta) | A study showed that Chaetogaster diaphanus consumed ostracods, but preyed more on cladocerans and copepods (which were more abundant) (Green 1954). |
| Polychaete worms (Annelida, Polychaeta) | Ostracods form a minor part of the diet of ragworms and amphinomid worms (Costa et al. 2006; Glynn 1984). |
| Leeches (Annelida, Hirudinae) | Occasional predators of ostracods (Young & Ironmonger 1980; Vandekerkhove et al. 2012). |
| Arrow worms (Chaetognatha) | Ostracods and copepods are the main prey of some species of these predatory marine worms (Froneman et al. 1998). |
| Tadpole shrimps (Crustacea, Branchiopoda, Notostraca) | Heterocypris incongruens has been found in the guts of Triops cancriformis, but copepods and cladocerans were more abundant (Boix et al. 2006). |
| Decapods (Crustacea, Decapoda) | Smaller marine mesopelagic decapod crustaceans feed on copepods and to a lesser extent ostracods. Ostracods also form part of the diet of marine prawns, freshwater crayfish and estuarine crabs (Foxton & Roe 1974; Robertson 1988; Reynolds & O'Keeffe 2005; Laughlin 1982). |
| Amphipods (Crustacea, Amphipoda) | Most amphipods are detritivores or scavengers, but predatory amphipods will eat ostracods (Dauby et al. 2001). |
| Bathynellaceans (Crustacea, Bathynellacea) | Some species of these blind, groundwater crustaceans prey on groundwater ostracods (Cho et al. 2006). |
| Copepods (Crustacea, Copepoda) | Ostracods form a small part of the diet of some species of freshwater copepods (Fryer 1957). |
| Other ostracods (Crustacea, Ostracoda) | Studies have shown that a small part of the diet of the marine giant ostracod Gigantocypris muelleri is made up of other ostracods, and marine planktonic halocyprid ostracods are consumed by Macrocypridina castanea. In non-marine habitats, Australocypris insularis was reported to feed on other smaller ostracod species, while Heterocypris incongruens is known to be cannibalistic (Kornicker 1969; Kornicker et al. 1976; Moguilevsky & Gooday 1977; Campbell 1995; Rossi et al. 2011). |
| Water mites (Arachnida, Acari) | A review listed 22 identified species of mites that prey on ostracods. Seven of these species prey only on ostracods (Davids et al. 1981; Proctor & Pritchard 1989). |
| Siphonophores (Cnidaria, Hydrozoa, Siphonophorae) | Many of these colonial hydrozoans are selective feeders, and one species (Hippopodius hippopus) feeds exclusively on ostracods (Purcell 1981). |
| Jellyfish (Cnidaria, Hydrozoa, Scyphozoa) | Ostracods are a prominent component of the diets of some species of jellyfish (Sørnes et al. 2008). |
| Anthozoans (Cnidaria, Anthozoa) | Ostracod remains have been recovered from both sea anemones and soft corals (Sebens & Koehl 1984). |
| Sea urchins (Echinodermata, Echinoidea) | A study showed that ostracods form part of the diet of a species of sea urchin, while another report noted that a sea urchin was gorged with ostracods (Neale 1983; Penchaszadeh et al. 2004). |
| Carnivorous plants | The bladderwort Utricularia preys on microcrustaceans, including ostracods (Harms 2002). |
Note that in some cases it is not clear if the consumption of ostracods is a result of predation or scavenging of already dead ostracods. For example, Baird (1850) noted that freshwater ostracods can be seen eating the dead carcasses of other ostracod species, but it is unclear if this is predatory or scavenging behaviour.
Ostracods have also been recovered from the stomachs of neon flying squids, but these are considered to be transit food items, i.e. not consumed directly, but were introduced into the squid stomachs from the stomachs of the squids' prey, such as plankton-eating fish (Nigmatullin et al. 2009).
Anti-predation strategies
1. Run (jump) away
Ostracods are generally very mobile, and will often try to escape danger either by swimming or crawling away.
Some species of the subfamily Cypricercinae have been observed preforming powerful jumps with their caudal rami, reaching speeds of 0.75 m/s. It is thought that such jumps are a predator response mechanism (Sars 1901; Matzke-Karasz et al. 2014).
The jumping ostracod Tanycypris centa
2. Hide
In experiments with juvenile flounder and ostracods from the Baltic Sea, it was noted that ostracods on an algal mat seem to be less predated on compared with those on a sand substrate (Aarnio & Mattila 2000). Other experiments have demonstrated that freshwater ostracods change their behaviour when fish are present, moving from open water to the relative safety of water plants (Roca et al. 1993; Uiblein et al. 1994; Kiss 2004). The colouration of some species of ostracods, especially ones with patches and stripes, acts as camouflage.
3. Live somewhere that predators can't tolerate.
Some species of ostracods, such as some giant ostracod species in Australia, live in saline lakes, with salinities too high to be tolerated by fish (Halse & McRae 2004). Other non-marine species are common in temporary water bodies, for example, seasonal lakes and rice fields. The dry periods of these habitats, tolerated by ostracods via their desiccation-resistant eggs, excludes some predators, such as fish. Small habitats, including springs and puddles, also exclude larger predators.
4. Light bomb the attacker
The myodocopid ostracod genus Vargula (sea firefly) is famous for its bioluminscence, used for mating and predator deterrence. If attacked by planktivorous fish, Vargula produce a bright and large (several cm) 'bomb-like' cloud of luminescence. Lasting for many seconds to more than a minute, this bright light probably startles and temporarily blinds the fish and sometimes causes the ostracod to be regurgitated. The luminescence can also attract larger fish, which in turn may predate on the initial predator. When attacked, the initial predator may eject the contents of its stomach, thus the Vargula can survive the encounter. Such predatory attacks on Vargula are reported to be rare, as fish appear to avoid them (Morin 1986).
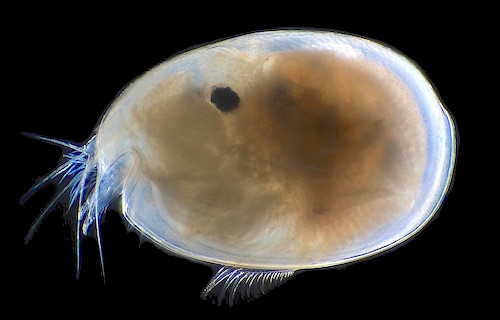
A female Vargula, length about 2.4 mm
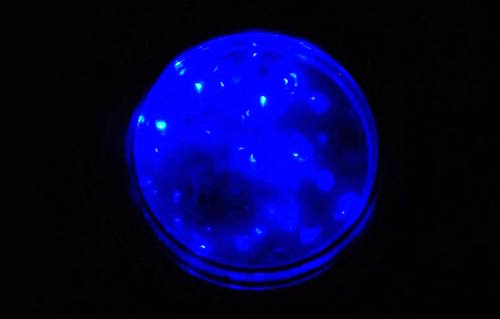
Bioluminescent Vargula in a small petri dish
5. Close the carapace and wait
If all else fails there is one last hope. Tightly close the carapace and wait. Very small fish may have a go at eating a passing ostracod, but are often rapidly spat out; the hard carapace is too tough for very young or small fish to crush. With larger fish and better developed teeth, ostracods may not fair so well.
If the ostracod can survive the gauntlet of the teeth intact it is possible for it to survive passage through the gut. Juvenile flounder in the Baltic Sea feed on ostracods (amongst other things) during the first few years of life, but up to 75% of ostracods eaten survive passage through the gut (Aarnio & Bonsdorff 1997). Other experiments have shown that freshwater ostracods can also reduce predation from fish by resisting digestion, with up to 26% emerging from the gut alive (Vinyard 1979).
Ostracods passing through the guts of tadpoles, waterfowl, and even rodents (only in experiments) intact and alive have also been reported (Lopez et al. 2002). This ability to survive passage through guts of various animals probably helps with dispersal (Brochet et al. 2010).
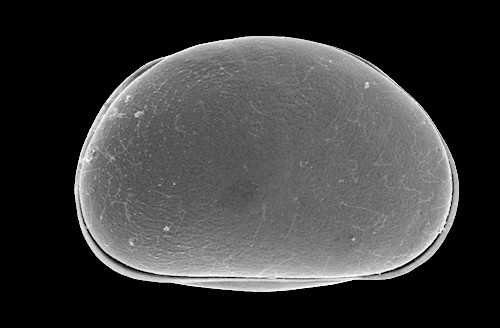
Side view of a tightly closed carapace.
Note how one valve overlaps the other, creating a tight seal.
References
Aarnio, K. & Bonsdorff, E. 1997. Passing the gut of juvenile flounder, Platichthys flesus: differential survival of zoobenthic prey species. Marine Biology, 129, 11-14.
Aarnio, K. & Mattila, J. 2000. Predation by juvenile Platichthys flesus (L.) on shelled prey species in a bare sand and a drift algae habitat. Hydrobiologia, 440, 347-355.
Alcalde, L., Derocco, N. N. & Rosset, S. D. 2010. Feeding in Syntopy: Diet of Hydromedusa tectifera and Phrynops hilarii (Chelidae). Chelonian Conservation and Biology, 9, 33-44.
Allen, J. R. M. & Wootton, R. J. 1984, Temporal patterns in diet and rate of food consumption of the three-spined stickleback (Gasterosteus aculeatus L.) in Llyn Frongoch, an upland Welsh lake. Freshwater Biology, 14, 335-346.
Arnold, R. J. & Convey, P. 1998. The life history of the diving beetle, Lancetes angusticollis (Curtis) (Coleoptera: Dytiscidae), on sub-Antarctic South Georgia. Polar Biology, 20, 153-160.
Baird, W. 1850. The Natural History of the British Entomostraca. Ray Society, London, 138-182, pls 18-23.
Baker, A. S. & McLachlan, A. J. 1979. Food preferences of Tanypodinae larvae (Diptera: Chironomidae). Hydrobiologia, 62, 283-288.
Blanco, S., Romo, S. & Villena, M.-J., 2004. Experimental study on the diet of mosquitofish (Gambusia holbrooki) under different ecological conditions in a shallow lake. Internationale Revue der gesamten Hydrobiologie und Hydrographie 89, 250-262.
Boix, D., Sala, J. Gascón, S. & Brucet, S. 2006. Predation in a temporary pond with special attention to the trophic role of Triops cancriformis (Crustacea: Branchiopoda: Notostraca). Hydrobiologia 571, 341-353.
Brochet, A. L., Gauthier-Clerc, M., Guillemain, M., Fritz, H., Waterkeyn, A., Baltanás, A. & Green, A. J. 2010. Field evidence of dispersal of branchiopods, ostracods and bryozoans by teal (Anas crecca) in the Camargue (southern France). Hydrobiologia 637, 255-261.
Brophy, T. E. 1980. Food habits of sympatric larval Ambystoma tigrinum and Notophthalmus viridescens. Journal of Herpetology, 14, 1-6.
Campbell, C. E. 1995. The influence of a predatory ostracod, Australocypris insularis, on zooplankton abundace and species composition in a saline lake. Hydrobiologia, 302, 229-239.
Campos, R. E. & Lounibos, L. P. 2000. Natural Prey and Digestion Times of Toxorhynchites rutilus (Diptera: Culicidae) in Southern Florida. Annals of the Entomological Society of America, 93, 1280-1287.
Cho, J., Park, J. & Reddy, Y. R. 2006. Brevisomabathynella gen. nov. with two new species from Western Australia (Bathynellacea, Syncarida): the first definitive evidence of predation in Parabathynellidae. Zootaxa, 1247, 25-42.
Costa, P. F., Oliveira, R. F. & da Fonseca, L. C. 2006. Feeding ecology of Nereis diversicolor (O. F. Müller) (Annelida, Polychaeta) on estuarine and lagoon environments in the southwest coast of Portugal. Pan-American Journal of Aquatic Sciences, 1, 114-126.
Culler, L. E. & Lamp, W. O. 2009. Selective predation by larval Agabus (Coleoptera: Dytiscidae) on mosquitoes: support for conservation-based mosquito suppression in constructed wetlands. Freshwater Biology, 54, 2003-2014.
Dauby, P., Scailteur, Y. & De Broyer, C. 2001. Trophic diversity within the eastern Weddell Sea amphipod community. Hydrobiologia, 443, 69-86.
Davids, C., Heijnis, C. F. & Weekenstroo, J. E. 1981. Habitat differentiation and feeding strategies in water mites in Lake Maarsseveen I. Aquatic Ecology, 15, 87-91.
Ernst, C. H. & Lovich, J. E. 2009. Turtles of the United States and Canada. The Johns Hopkins University Press, Maryland.
Foxton, P. & Roe, H. S. J. 1974. Observations on the nocturnal feeding of some mesopelagic decapod Crustacea. Marine Biology, 28, 37-49.
Froneman, P. W., Pakhomov, E. A., Perissinotto, R. & Meaton, V. 1998. Feeding and predation impact of two chaetognath species, Eukrohnia hamata and Sagitta gazellae, in the vicinity of Marion Island (southern ocean). Marine Biology, 131, 95-101.
Fryer, G. 1957. The food of some freshwater cyclopoid copepods and its ecological significance. Journal of Animal Ecology, 26, 263-286.
Ghioca-Robrecht, D. M. & Smith, L. M., 2008. Feeding ecology of polymorphic larval barred tiger salamanders in playas of the Southern Great Plains. Canadian Journal of Zoology, 86, 554-563.
Glynn, P. W. 1984. An amphinomid worm predator of the crown-of-thorns sea star and general predation on asteroids in eastern and western Pacific coral reefs. Bulletin of Marine Science, 35, 54-71.
Gordon, J. D. M., Nishida, S. & Nemoto, T. 1985. The diet of mesopelagic fish from the Pacific coast of Hokkaido, Japan. Journal of the Oceanographical Society of Japan, 41, 89-97.
Green, J. 1954. A note on the food of Chaetogaster diaphanus. Journal of Natural History Series 12, 7, 842-844.
Griffiths, H. I., Martin, D. S., Shine, A. J. & Evans, J. G. 1993. The ostracod fauna (Crustacea, Ostracoda) of the profundal benthos of Loch Ness. Hydrobiologia, 254, 111-117.
Halse, S. A. & McRae, J. M. 2004. New genera and species of 'giant' ostracods (Crustacea: Cyprididae) from Australia. Hydrobiologia, 524, 1-52.
Harding, J. P. 1962. Mungava munda and four other new species of ostracod crustaceans from fish stomachs. The Natural History of Rennell Island, British Solomon Islands, 4, 51-62.
Harms, S. 2002. The effect of bladderwort Utricularia predation on microcrustacean prey. Freshwater Biology, 47, 1608-1617.
Havel, J., Link, J. & Niedzwiecki, J. 1993. Selective predation by Lestes (Odonata, Lestidae) on littoral microcrustacea. Freshwater Biology, 29, 47-58.
Hayashi, M. & Ohba, S-Y. 2018. Mouth morphology of the diving beetle Hyphydrus japonicus (Dytiscidae: Hydroporinae) is specialized for predation on seed shrimps. Biological Journal of the Linnean Society, 125 (2), 315-320.
Henderson, P. A. 1990. Freshwater Ostracods. In: Kermack, D. M. & Barnes, R. S. K., Synopses of the British Fauna (New Series), 42, pp. 228.
Kiss, A. 2004. Field and laboratory observations on the microhabitat and food selection as well as predator avoidance of Notodromas monacha (Crustacea: Ostracoda). Revista Española de Micropaleontología, 36, 147-156.
Kornicker, L. S. 1969. Morphology, ontogeny, and intraspecific variation of Spinacopia, a new genus of myodocopid ostracod (Sarsiellidae). Smithsonian Contributions to Zoology, 8, 1-50.
Kornicker, L. S., Wirsing, S. & McManus, M. 1976. Biological Studies of the Bermuda Ocean Acre: Planktonic Ostracoda. Smithsonian Contributions to Zoology, 223, 1-34.
Laughlin, R. A. 1982. Feeding habits of the blue crab, Callinectes sapidus Rathbun, in the Apalachicola Estuary, Florida. Bulletin of Marine Science, 32, 807-822.
Leal, J. H. 2008. A remarkable new genus of carnivorous, sessile bivalves (Mollusca: Anomalodesmata: Poromyidae) with descriptions of two new species. Zootaxa, 1764, 1-18.
Legner, E. F., Tsai, T. C. & Medved, R. A. 1976. Environmental stimulants to asexual reproduction in the planarian, Dugesia dorotocephala. Enthomophaga, 21, 415-423.
Lilly, C. K., Ashley, D. L. & Tarter, D. C. 1978. Observations on a population of Sialis itasca Ross in West Virginia (Megaloptera: Sialidae). Psyche, 85, 209-218.
Lopez, L. C. S., Gonçalves, D. A., Mantovani, A. & Rios, R. I. 2002. Bromeliad ostracods pass through amphibian (Scinax perpusillus) and mammalian guts alive. Hydrobiologia, 485, 209-211.
Lowndes, A. G. 1930. Living ostracods in the rectum of a frog. Nature, 126, 958.
Maddocks, R. F. 1988. One hundred million years of predation on ostracods: the fossil record in Texas. In: Hanai, T., Ikeya, N. & Ishizaki, K. (eds). Evolutionary Biology on Ostracoda, Proceedings of the Ninth international Symposium on Ostracoda. Kodansha Ltd., Tokyo, Japan, 637-657.
Matzke-Karasz, R., Nagler, C. & Hofmann, S. 2014. The ostracod springtail - camera recordings of a previously undescribed high-speed escape jump in the genus Tanycypris (Ostracoda, Cypridoidea). Crustaceana, 87, 1072-1094.
Mbahinzireki, G, Uiblein, F. & Winkler, H. 1991. Microhabitat selection of ostracods in relation to predation and food. Hydrobiologia, 222, 115-119.
Moguilevsky, A. & Gooday, A. J. 1977. Some observations on the vertical distribution and stomach contents of Gigantocypris muelleri Skogsberg 1920 (Ostracoda, Myodocopina). In: Loffler, H. & Danielopol, D. (eds) Aspects of ecology and zoogeography of recent and fossil Ostracoda. Junk, The Hague, 263-270.
Morin, J. G. 1986. Firefleas of the Sea: Luminescent Signaling in Marine Ostracode Crustaceans. The Florida Entomologist, 69, 105-121.
Neale, J. W. 1983. The Ostracoda and uniformitarianism. I. The later record: Recent, Pleistocene and Tertiary. Proceedings of the Yorkshire Geological Society, 44, 305-326.
Nigmatullin, C. M., Shchetinnikov, A. S. & Shukhgalter, O. A. 2009. On feeding and helminth fauna of neon flying squid Ommastrephes bartramii (Lesueur, 1821) (Cephalopoda: Ommastrephidae) in the southeastern Pacific. Revista de Biología Marina y Oceanografía 44, 227-235.
Papácek, M. 2001. Small aquatic and ripicolous bugs (Heteroptera: Nepomorpha) as predators and prey: The question of economic importance. European Journal of Entomolology, 98, 1-12.
Penchaszadeh, P. E., Bigatti, G. & Miloslavich, P. 2004. Feeding of Pseudechinus magellanicus (Philippi, 1857) (Echinoidea: Temnopleuridae) in the SW Atlantic Coast (Argentina). Ophelia, 58, 91-99.
Proctor, H. & Pritchard, G. 1989. Neglected predators: water mites (Acari: Parasitengona: Hydrachnellae) in Freshwater Communities. Journal of the North American Benthological Society, 8, 100-111.
Proctor, V. W. 1964. Viability of crustacean eggs recovered from ducks. Ecology, 45, 656-658.
Purcell, J. E., 1981. Dietary composition and diel feeding patterns of epipelagic siphonophores. Marine Biology, 65, 83-90.
Reyment, R. A. 1966. Preliminary observations on gastropod predation in the Western Niger Delta. Palaeogeography, Palaeoclimatology, Palaeoecology, 2, 81-102.
Reyment, R. A. & Elewa, A. M. T. 2003. Predation by drills on Ostracoda. In: Predator - prey interactions in the fossil record. Topics in Geobiology, 20, 93-111.
Reynolds, J. D. & O'Keeffe, C. 2005. Dietary patterns in stream- and lake-dwelling populations of Austropotamobius pallipes. Bulletin Français de la Pêche et de la Pisciculture 376/377, 715-730.
Robertson, A. I. 1988. Abundance, diet and predators of juvenile banana prawns, Penaeus merguiensis, in a tropical mangrove estuary. Australian Journal of Marine and Freshwater Research, 39,467-478.
Roca, J. R., Baltanás, A. & Uiblein, F. 1993. Adaptive responses in Cypridopsis vidua (Crustacea: Ostracoda) to food and shelter offered by a macrophyte (Chara fragilis). Hydrobiologia, 262, 127-131.
Rossi, V., Benassi, G., Belletti, F. & Menozzi, P. 2011. Colonization, population dynamics, predatory behaviour and cannibalism in Heterocypris incongruens (Crustacea: Ostracoda). Journal of Limnology, 70, 102-108.
Sars, G.O. 1901. Contributions to the knowledge of the fresh-water Entomostraca of South America, as shown by artificial hatching from dried material. Archiv for Mathematik og Naturvidenskab, 24, 3–46.
Sebens, K. P. & Koehl, M. A. R. 1984. Predation on zooplankton by the benthic anthozoans Alcyonium siderium (Alcyonacea) and Metridium senile (Actiniaria) in the New England subtidal. Marine Biology, 81, 255-271.
Smith, R. J. & Kamiya, T. 2008. The ontogeny of two species of Darwinuloidea (Ostracoda, Crustacea). Zoologischer Anzeiger, 247, 275-302.
Sohn, I. G. & Chatterjee, S. 1979. Freshwater Ostracodes from Late Triassic Coprolite in Central India. Journal of Paleontology, 53, 578-586.
Sørnes, T. A., Hosia, A., Båmstedt, U. & Aksnes, D. L. 2008. Swimming and feeding in Periphylla periphylla (Scyphozoa, Coronatae). Marine Biology, 153, 653-659.
Spencer, M. & Blaustein, L. 2001. Risk of predation and hatching of resting eggs in the ostracod Heterocypris incongruens. Journal of Crustacean Biology, 21, 575-581.
Thompson, E. M & Tsuji, F. I. 1989. Two populations of the marine fish Porichthys notatus, one lacking in luciferin essential for bioluminescence. Marine Biology, 102, 161-165.
Tszydel, M. & Grzybkowska, M. 2011. Diet and secondary production of some species of Hydropsyche larvae (Trichoptera: Hydropsychidae) in the tailwater affected by dam removal and the emptying of an impoundment. Zoosymposia, 5, 465-480.
Uiblein, F., Roca, J. R. & Danielopol, D. L. 1994. Experimental observations on the behaviour of the ostracode Cypridopsis vidua. Verhandlungen des Internationalen Verein Limnologie, 25, 2418-2420.
Vandekerhove, J., Namiotko, T., Hallmann, E. & Martens, K. 2012. Predation by macroinvertebrates on Heterocypris incongruens (Ostracoda) in temporary ponds: impacts and responses. Fundamental and Applied Limnology, 181, 39-47.
Victor, R., Chan, G. L. & Fernando, C. H. 1979. Notes on the recovery of live ostracods from the gut of the white sucker (Catostomus commersoni Lacépède) 1808, (Pisces: Catostomidae). Canadian Journal of Zoology, 57, 1745-1747.
Vinyard, G. 1979. An ostracod (Cypridopsis vidua) can reduce predation from fish by resisting digestion. American Midland Naturalist, 102, 108-190.
Vodopich, D. S. & Cowell, B. C. 1984. Interaction of factors governing the distribution of a predatory aquatic insect. Ecology, 65, 3-52.
Young, J. O. & Ironmonger, J. W. 1980. A laboratory study of the food of three species of leeches occurring in British lakes. Hydrobiologia, 68, 209-215.
